Development and Assessment of a Self-Nanoemulsifying Drug Delivery System Containing Bilastine: An Effective Strategy to Improve Dissolution
DOI:
https://doi.org/10.54133/ajms.v9i2.1874Keywords:
Bilastine, Drug delivery system, Dissolution, Self nano-emulsionAbstract
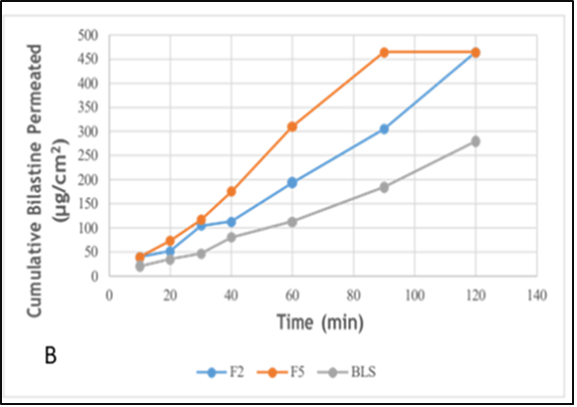
Background: Innovative approaches, including self-nanoemulsifying drug delivery systems, have the potential to solve a variety of drug formulation challenges, such as solubility, stability, and bioavailability. The drug candidate in this research is the strong and extremely selective H1-antihistamine, bilastine, that belongs to BCS II with low solubility and high permeability. Objective: This research aims to formulate an oral self-nanoemulsion of bilastine to improve dissolution. Methods: A total of fifteen liquid self-nanoemulsion drug delivery systems (SNEDDS) formulas were developed using oleic acid, cremophor, and Transcutol as the appropriate oils, stabilizers, and co-stabilizers based on the solubility studies of bilastine in various oils, stabilizers, and co-stabilizers. We used pseudoternary phase diagrams to look at the behavior of the component phases and the area of the nanoemulsion. The physicochemical properties of the formulas, in addition to thermodynamic stability, droplet size, polydispersity index, zeta potential, self-emulsification time, drug content, and durability of the developed formulations, were assessed. Results: This study demonstrated that formula 5, with a 20% oleic acid, 27% cremophor, and 53% Transcutol composition, had lower globular size, acceptable drug content, better in-vitro drug release, and ex-vivo permeability characteristics than pure bilastine powder. Conclusions: The mentioned factors all point to the preparation of self-nanoemulsifying drug delivery systems as a potential means for improving the dissolution of poorly soluble drugs like bilastine.
Downloads
References
Nair AB, Singh B, Shah J, Jacob S, Aldhubiab B, Sreeharsha N, et al. Formulation and evaluation of self-nanoemulsifying drug delivery system derived tablet containing sertraline. Pharmaceutics. 2022;14(2):336. doi: 10.3390/pharmaceutics14020336.
Dolly SA, Talele AN, Prajapati AP, Narkhede SB. Formulation development and evaluation of sublingual drug delivery system of bilastine for allergic rhinoconjunctivitis. IAJPS. 2021;8(4):166-181. doi: 10.5281/zenodo.4710585.
Falcon RM, Caoili SE. Immunologic, genetic, and ecological interplay of factors involved in allergic diseases. Front Allergy. 2023;4:1215616. doi: 10.3389/falgy.2023.1215616.
Leceta A, Sologuren A, Valiente R, Campo C, Labeaga L. Bilastine in allergic rhinoconjunctivitis and urticaria: a practical approach to treatment decisions based on queries received by the medical information department. Drugs Context. 2017;6:212500. doi: 10.7573/dic.212500.
Zhang K, Wang Q, Yang Q, Wei Q, Man N, Adu-Frimpong M, et al. Enhancement of oral bioavailability and anti-hyperuricemic activity of isoliquiritigenin via self-microemulsifying drug delivery system. AAPS Pharm SciTech. 2019;20:1. doi: 10.1208/s12249-019-1421-0.
Tong Y, Zhang Q, Shi W, Wang J. Mechanisms of oral absorption improvement for insoluble drugs by the combination of phospholipid complex and SNEDDS. Drug Deliv. 2019;26(1):1155-1166. doi: 10.1080/10717544.2019.1686086.
Rathore C, Hemrajani C, Sharma AK, Gupta PK, Jha NK, Aljabali AA, et al. Self-nanoemulsifying drug delivery system SNEDDS mediated improved oral bioavailability of thymoquinone: Optimization, characterization, pharmacokinetic, and hepatotoxicity studies. Drug Deliv Transl Res. 13(1), 292-307. doi: 10.1007/s13346-022-01193-8.
Hazzaa SA, Abd-Alhameed SN. Formulation and evaluation of optimized Zaltoprofen lyophilized tablets by Zydis technique. Iraqi J Pharm Sci. 2017;26(1):40-49. doi: 10.31351/vol26iss1pp40-49.
Ghareeb MM, Neamah AJ. Formulation and characterization of nimodipine nanoemulsion as ampoule for oral route. Int J Pharm Sci Res. 2017;8(2):591. doi: 10.13040/IJPSR.0975-8232.8(2).591-02.
Nasr AM, Gardouh AR, Ghonaim HM, Ghorab MM. Design, formulation and in-vitro characterization of Irbesartan solid self-nanoemulsifying drug delivery system S-SNEDDS prepared using spray drying technique. J Chem Pharm Res. 2016;8(2):159-183.
Abushal AS, Aleanizy FS, Alqahtani FY, Shakeel F, Iqbal M, Haq N, et al. Self-nanoemulsifying drug delivery system SNEDDS of apremilast: In vitro evaluation and pharmacokinetics studies. Molecules. 2022;27(10):3085. doi: 10.3390/molecules27103085.
Mahmoud H, Al-Suwayeh S, Elkadi S. Design and optimization of self-nanoemulsifying drug delivery systems of simvastatin aiming dissolution enhancement. Afr J Pharm Pharmacol. 2013;7(22):1482-1500. doi: 10.5897/AJPP2013.2987.
Al-Tamimi DJ, Hussein AA. Formulation and characterization of self-microemulsifying drug delivery system of tacrolimus. Iraqi J Pharm Sci. 2021;30(1):91-100. doi: 10.31351/vol30iss1pp91-100.
Alothaid H, Aldughaim MS, Yusuf AO, Yezdani U, Alhazmi A, Habibullah MM, et al. A comprehensive study of the basic formulation of supersaturated self-nanoemulsifying drug delivery systems SNEDDS of albendazolum. Drug Deliv. 2021;28(1):2119-2126. doi: 10.1080/10717544.2021.1986601.
Chandan C, Maheshwari RK. Mixed solvency concept in reducing stabilizer concentration of self-emulsifying drug delivery systems of candesartan cilexetil using D-optimal mixture design. AJP. 2013;7(2). doi: 10.4103/0973-8398.115960.
Sabri LA, Hussein AA. Comparison between conventional and supersaturable self-nanoemulsion loaded with nebivolol: Preparation and in-vitro/ex-vivo evaluation. Iraqi J Pharm Sci. 2020;29(1):216-225. doi: 10.31351/vol29iss1pp216-225.
Ali HH, Hussein AA. Oral nanoemulsions of candesartan cilexetil: Formulation, characterization and in vitro drug release studies. AAPS Open. 2017;3(1):4. doi: 10.1186/s41120-017-0016-7.
Bhagwat DA, Swami PA, Nadaf SJ, Choudhari PB, Kumbar VM, More HN, et al. Capsaicin loaded solid SNEDDS for enhanced bioavailability and anticancer activity: In-vitro, in-silico, and in-vivo characterization. J Pharm Sci, 2021;110(1):280-291. doi: 10.1016/j.xphs.2020.09.066.
Ochoa D, Román M, Belmonte C, Martín-Vilchez S, Mejía-Abril G, Abad-Santos F, et al. Pharmacokinetics and safety of a bilastine once-daily, preservative-free, ophthalmic formulation. Adv Ther. 2021;38(7):4070-408. doi: 10.1007/s12325-021-01801-y.
Salim FF, Rajab NA. Formulation and characterization of piroxicam as self-nano emulsifying drug delivery system. Iraqi J Pharm Sci. 2020;29(1):174-183. doi: 10.31351/vol29iss1pp174-183.
Teaima M, Hababeh S, Khanfar M, Alanazi F, Alshora D, El-Nabarawi M. 2022. Design and optimization of pioglitazone hydrochloride self-nanoemulsifying drug delivery system SNEDDS incorporated into an orally disintegrating tablet. Pharmaceutics. 2022;14(2):425. doi: 10.3390/pharmaceutics14020425.
Verma R, Kaushik A, Almeer R, Rahman MH, Abdel-Daim MM, Kaushik D. 2021. Improved pharmacodynamic potential of rosuvastatin by self-nanoemulsifying drug delivery system: An in vitro and in vivo evaluation. Int J Nanomedicine, 2021;16:905-922. doi: 10.2147/IJN.S311078.
Shrestha B, Dunn L. The declaration of Helsinki on medical research involving human subjects: A review of seventh revision. J Nepal Health Res Counc. 2020;17(4):548-552. doi: 10.33314/jnhrc.v17i4.1042.
Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, et al. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 2011.
Huang YB, Tsai YH, Yang WC, Chang JS, Wu PC. Guidance for industry, dissolution testing of immediate release solid oral dosage forms. Biol Pharm Bull. 2004;27(10):1626-1629. doi: 10.1155/2013/197398.
Čebzan D, Pavlović A, Kokanović J, Trijić S, Jovanović V, Mladenović A, et al. Dissolution profile comparison using similarity factor F2 in sertralin tablets. Dissolution Technol. 2006;56(5):694-695. doi: 10.14227/DT060399P15.
Rathi SG, Chaudhari DB, Vaghela SS, Kamani KR. Physicochemical characterization and dissolution enhancement of bilastine by solid dispersion. Int J Pharm Sci Rev Res. 2021;69(1):194-200. doi: 10.47583/ijpsrr.2021.v69i01.028.
Khatri P, Shao J. Mechanism and structural factors of lipid and stabilizer in the formation of self-emulsified nanoemulsion. J Pharm Sci. 2018;107(8):2198-2207. doi: 10.1016/j.xphs.2018.03.017.
Jain K, Kumar RS, Sood S, Gowthamarajan K. Enhanced oral bioavailability of atorvastatin via oil-in-water nanoemulsion using aqueous titration method. J Pharm Sci Res. 2013;5(1):18.
Amin MM, El Gazayerly ON, Abd El-Gawad NA, Abd El-Halim SM, El-Awdan SA. Effect of formulation variables on design, in vitro evaluation of valsartan SNEDDS and estimation of its antioxidant effect in adrenaline-induced acute myocardial infarction in rats. Pharm Dev Technol. 2016;21(8):909-920. doi: 10.3109/10837450.2016.1162630.
Usta DY, Timur B, Teksin ZS. Formulation development, optimization by Box-Behnken design, characterization, in vitro, ex-vivo, and in vivo evaluation of bosentan-loaded self-nanoemulsifying drug delivery system: A novel alternative dosage form for pulmonary arterial hypertension treatment. Eur J Pharm Sci. 2022;174:106159. doi: 10.1016/j.ejps.2022.106159.
Inugala S, Eedara BB, Sunkavalli S, Dhurke R, Kandadi P, Jukanti R, et al. Solid self-nanoemulsifying drug delivery system S-SNEDDS of darunavir for improved dissolution and oral bioavailability: In vitro and in vivo evaluation. Eur J Pharm Sci. 2015;74:1-10. doi: 10.1016/j.ejps.2015.05.001.
Saifullah S, Kanwal T, Ullah S, Kawish M, Habib SM, Ali I, et al. Design and development of lipid modified chitosan containing muco-adhesive self-emulsifying drug delivery systems for cefixime oral delivery. Chem Phys Lipids. 2021;235:105052. doi: 10.1016/j.chemphyslip.2021.105052.
Mekhilef SF, Hussein AA. 2018. Novel combination for self-nanoemulsifying drug delivery system of candesartan cilexetil. Iraqi J Pharm Sci. 2018;27(2):123-134. doi: 10.31351/vol27iss2pp123-134.
Fitria A, Hanifah S, Chabib L, Uno AM, Munawwarah H, Atsil N, et al. 2021. Design and characterization of propolis extract loaded self-nano emulsifying drug delivery system as immunostimulant. Saudi Pharm J. 2021;29(6):625-634. doi: 10.1016/j.jsps.2021.04.004.
Villalobos-Hernández JR, Müller-Goymann CC. Novel nanoparticulate carrier system based on carnauba wax and decyl oleate for the dispersion of inorganic sunscreens in aqueous media. Eur J Pharm Biopharm. 2005;60(1):113-122. doi: 10.1016/j.ejpb.2004.11.002.
Reddy MR, Gubbiyappa KS. Formulation development, optimization and characterization of Pemigatinib-loaded supersaturable self-nanoemulsifying drug delivery systems. Future J Pharm Sci. 2022;8(1):45. doi: 10.1186/s40502-022-00367-7.
The United States Pharmacopoeia USP 30 N. NF 25. 2006. The United States Pharmacopeial Convention Inc. USA: Rockville, 1191-1193, 2110.
Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63(3):288-294. doi: 10.1016/j.ejpb.2006.02.003.
Bakhle SS, Avari JG. Development and characterization of solid self-emulsifying drug delivery system of cilnidipine. Chem Pharm Bull. 2015;63(6):408-417. doi: 10.1248/cpb.c14-01047.
Park EJ, Choi SA, Min KA, Jee JP, Jin SG, Cho KH. Development of alectinib-suspended SNEDDS for enhanced solubility and dissolution. Pharmaceutics, 2022;14(8):1694. doi: 10.3390/pharmaceutics14081694.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











